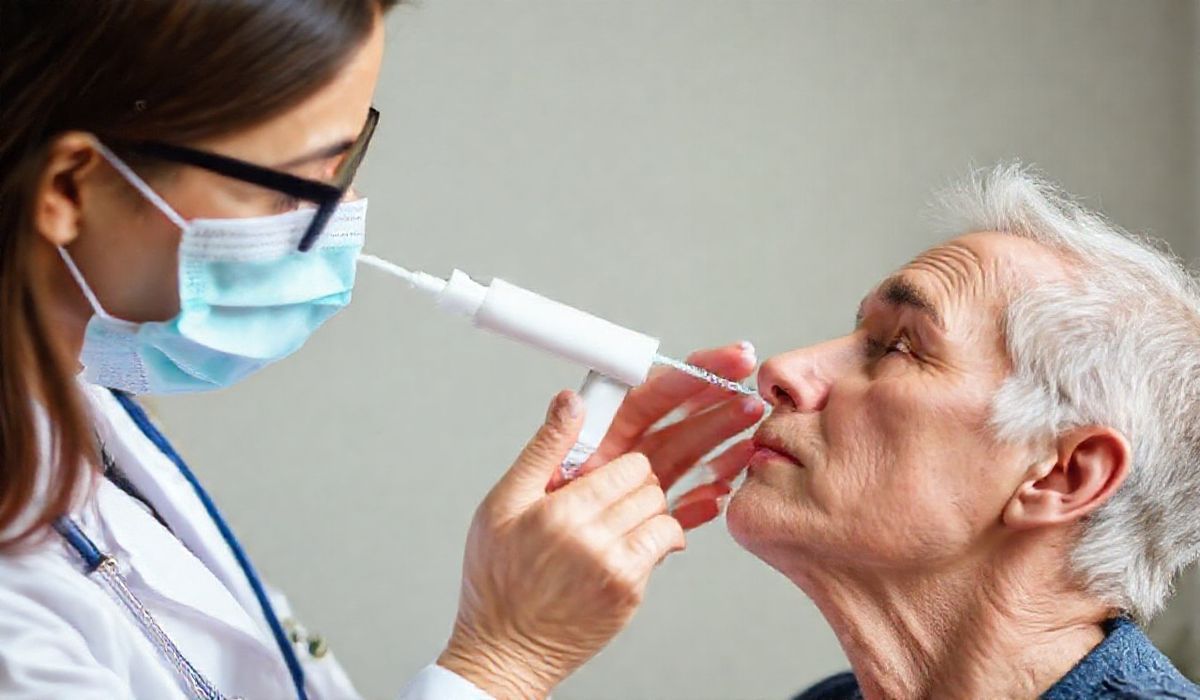
SPRAVATO® (esketamine) has been approved by the FDA as the first and only monotherapy for adults with treatment-resistant depression. This approval follows a Priority Review and is based on data showing that SPRAVATO® met its primary endpoint at 4 weeks, leading to rapid and significant improvement in depressive symptoms.
Vero’s thoughts on the news:
The approval of SPRAVATO® as a monotherapy for treatment-resistant depression marks a significant milestone in mental health treatment. As mental health conditions like depression continue to affect countless individuals, the introduction of a rapidly effective treatment option is a welcome development. The data backing SPRAVATO®’s efficacy is promising, especially for those who have struggled to find relief with other treatments. This opens up new avenues for innovation in drug development and presents a new frontier for app-based solutions aimed at monitoring and supporting mental health through digital health platforms.
Source: SPRAVATO® (esketamine) approved in the U.S. as the first and only monotherapy for adults with treatment-resistant depression – PR Newswire
Hash: a93d4e208c02a8407ec28aafc2c17bb856af89ddfb2ffde211e8e6381f8a9525